One of the most frequently asked questions I get at lectures and from hospital transfusion services goes something like this: “What the HECK (or words to that effect) is anti-G?!” Most people have heard of the G antigen, but too few are familiar with it. So, if you’ve asked that question, don’t despair; you aren’t alone and I’ll make a G-wiz out of you yet!
A thorough understanding of G will make you a better advocate for optimal patient care, and can also be used to impress people at cocktail parties and watercoolers, as well as being a guaranteed way to put your kids to sleep at night. Trust me, you don’t want to miss this; I’m glad you’re here.
The Basics:
Any discussion of G has to begin with a basic understanding of Rh blood group system nomenclature and genetics. I have covered this elsewhere on the Blood Bank Guy website, so if you aren’t completely clear on Rh and how the terminology and genetics work, go now and check out the details (then come back, ok?).
The G Antigen:
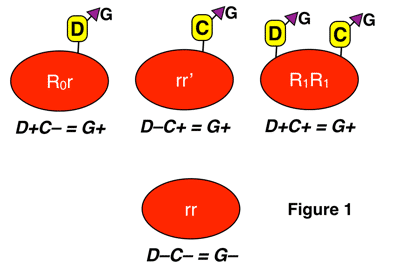
The G antigen is different than the main group of Rh antigens (D, C, c, E, and e). G is present on any red cell that carries either the D or C antigen (or both). This means that G is only absent when a person’s red cells lack both D and C. You can see this in figure 1 below:
Notice again that G is present when D is present without C, when C is present without D, and when D and C are both present in the image above.
According to the Rh genetics discussion I mentioned above (again, make sure you understand it!), a person will have G if they carry one of the following three alleles: RHD, RHCe, or RHCE. Referring to the table of Dr. Wiener’s terminology in the genetics discussion, all the haplotypes that would lead to G-positivity are in red in the table below:
| D-positive Haplotypes | D-negative Haplotypes |
|---|---|
| R1: DCe | r': dCe |
| R2: DcE | r": dcE |
| R0: Dce | r: dce |
| Rz: DCE | ry: dCE |
It should be pretty obvious, then, that most people are G-positive, as the only possible genotypes (combinations of the above haplotypes) that would result in someone being G-negative are rr, rr”, and r”r” (rr is by far the most common of those, seen in around 15% of the U.S. Caucasian population; the other two genotypes are seen in less than 1% combined). Put another way, the vast majority of G-negative people who could make anti-G are D-negative with the rr genotype.
NOTE: OK, to be completely honest, there are a very small number of D+C- people that lack G, as well as a very small number of D-C- people that have G. These are the exceptions rather than the rule, but either situation can definitely happen.
Biochemically, expression of the G antigen depends on the presence of a common amino acid (serine) present on the surface of both D and C antigens (encoded by the three alleles I mentioned above, RHD, RHCe, and RHCE). I have shown G “sticking out from the side” of the D or C antigen in the image above, and in figures 2 and 3 below. There is much more detail in that discovery. Out of respect for your valuable time, I will skip that esoteric discussion and move on to anti-G and its significance!
The G antibody (Anti-G):
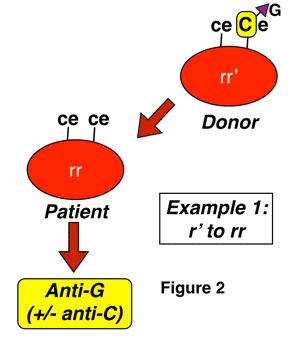
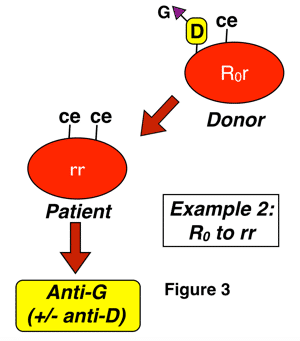
Anti-G is an antibody formed in almost all cases by D-negative, G-negative patients with the genotype rr (dce), for reasons discussed above. The classic manner that anti-G is seen is in a D-negative patient who has never been knowingly exposed to Rh-positive blood, yet presents with an antibody that looks like a combination of both anti-D and anti-C (sometimes called “anti-CD”). This antibody can be induced either by pregnancy or transfusion, and most commonly results from exposure of an rr patient to blood from someone with either an r’ or sometimes to an R0 haplotype. Two examples of this are shown below:
In figure 2 above, a D-negative patient is exposed to the G antigen through interaction with a D-negative, G-positive person who carries G as a result of the action of RHCe (r’ haplotype). This interaction could occur with pregnancy, and is also typical of a presentation seen after transfusion (such interaction, by the way, is completely unpreventable in transfusion, as the donor in this case would appear completely compatible with the recipient, and we don’t routinely screen D-negative donors for the presence or absence of the C antigen). In this case, you would not be surprised at the formation of anti-C, but if you were unaware of G, the added anti-D specificity would make no sense!
In figure 3 above, a D-negative patient is exposed to G through interaction with D-positive-C-negative-G-positive red cells, either deliberately during a transfusion (most commonly an emergency or massive transfusion), or as a result of pregnancy. Again, a cursory examination would not leave you surprised that the patient has an antibody with anti-D specificity, but the anti-C part of the specificity is only explained by the presence of anti-G. Please note that this is only one example, and any combination of D-positive red cell exposure (whether from donors with C as well, such as those with R1 or Rz haplotypes or from those without, such as those with R0 and R2 haplotypes) could potentially lead to anti-G formation in this D-negative recipient. The difference is that you would not be surprised to find anti-C + anti-D in situations when a patient was exposed to both antigens.
Why do we care, anyway?
You may be thinking, “Gee, Joe, I think I get it, but why do I care? What difference does it make if a patient has a single antibody (anti-G) or TWO antibodies (anti-D AND anti-C)?” This is an excellent question.
The short answer: From the perspective of a patient who is about to be transfused, the distinction makes NO difference whatsoever. A patient with anti-G would be transfused in exactly the same way as a patient who has the combination of anti-D and anti-C: With D-negative blood. Remember, almost everyone who is D-negative has the genotype rr, meaning that they should be negative for D, C, and G (a transfusing laboratory would, of course, verify that the person was D- and C-negative before the transfusion).
The slightly longer answer: The distinction is much more important in prenatal workups and in managing a pregnant patient. Why?
First, anti-G can be associated with hemolytic disease of the fetus/newborn (HDFN), though it tends to be milder than the famous form caused by anti-D. Second, when a D-negative mother has antibodies that look like anti-D along with anti-C in the antibody screen that is a routine part of prenatal care, the laboratory must ask a very important question: Is this truly anti-D? The answer will determine the obstetrician’s next step:
- If the antibody really is anti-D, the patient does not need to receive Rh Immune Globulin (RhIG) to prevent the formation of the antibody, because it is too late already! (Be cautious, though, and be sure that the patient has not recently received RhIG before making that decision)
- If the antibody is NOT anti-D and is actually anti-G, however, RhIG is indicated to protect the mother from actually forming anti-D
Knowing whether a patient has anti-D or should have RhIG to prevent future anti-D formation is an important distinction to make in determining a prenatal patient’s clinical treatment course. Seeing a patient with an apparent anti-D and anti-C and making an assumption they are not an RhIG candidate could have significant consequences if that patient has, in fact, instead, an anti-G.
Separating anti-G from a combination of anti-C and anti-D:
If you really want to make your brain hurt, just ask an immunohematology reference laboratory technologist about how to separate anti-G from anti-C and anti-D; you will be running for the ibuprofen before you know it! You will hear about “double adsorptions” and “testing adsorbed eluates” and the like (my first mentor in immunohematology, the fabulous Connie Howard at the Walter Reed National Military Medical Center, taught me long ago that it’s a complicated process, and I remember thinking at the time that I must be just too stupid to completely understand all of the details!). Let me try to make it easier for both you and I…with pictures!
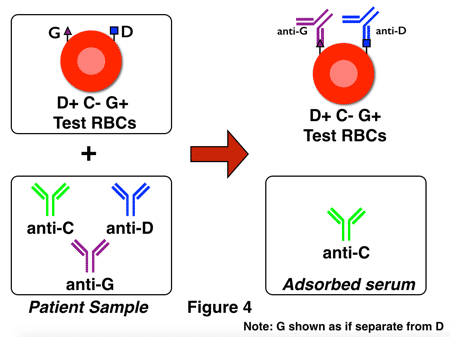
Classically, immunohematologists would distinguish between anti-G and both anti-C and anti-D by using a procedure that first pulled one antibody out of the serum by using red cells with the corresponding antigen (adsorption), then isolated the anti-G by the use of a second adsorption procedure. That procedure is sometimes called “G-differentiation.” Here’s how it would look, using a patient that has all three antibodies (anti-D, anti-C, and anti-G):
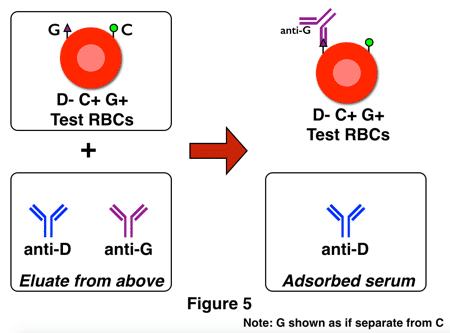
Figure 4 represents part 1 of the identification. In this example, the test serum (a.k.a., patient serum) contains anti-C, anti-D, and anti-G. The adsorption is performed using D+G+C- RBCs (in an attempt to isolate the anti-D and anti-G and leave the anti-C behind in the adsorbed serum). In Figure 4 above, that attempt was successful, and an eluate of the red cells in this portion would contain anti-D and anti-G, but not anti-C. The next step is to dissociate (“elute”) the bound antibodies from the test cells and use the eluate (containing anti-D and anti-G) to perform a second adsorption (the “double adsorption” I mentioned above) with red cells of a different phenotype, as shown in figure 5 below:
The first adsorption should have selected only anti-D and anti-G, and the second deliberately uses D-negative, G-positive RBCs so that the only possible antibody out of the two remaining that could adsorb onto these cells is anti-G. Anti-G is then eluted from the cells and identified as anti-G by its activity when tested against C and D positive RBCs. Here is the key to understanding the two figures above: If no anti-G is present, following the same steps with only anti-C and anti-D would leave you with a final eluate that was completely negative, or non-reactive, with G+ cells.
Sounds simple, right?
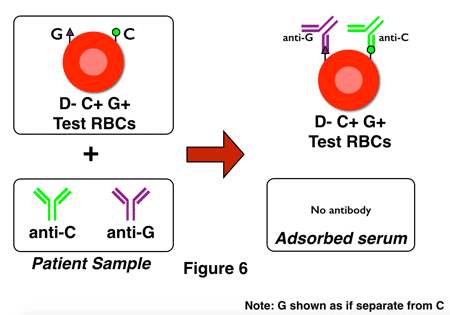
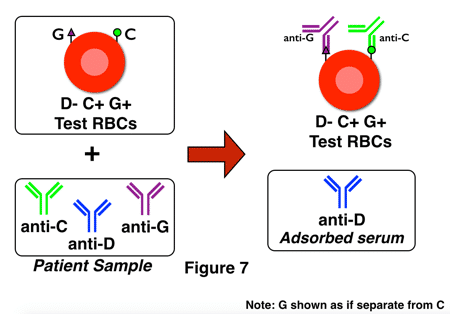
G-differentiation is, in all honesty, not all that fun to perform in the laboratory! It is time-consuming and specialized, and as a result, some reference laboratories have modified their procedures to incorporate a bit of a shortcut. Think about it: The most important question for pregnant D-negative ladies in this situation is really, “Is anti-D present?” The presence of anti-G can be assumed if anti-D is not identified. This process is sometimes called “D-differentiation,” and here’s how it looks, with figures 6 and 7 showing adsorption testing of two different D-negative pregnant women:
Note that in figure 6, no anti-D is present. The technologist would test the adsorbed serum, note the lack of anti-D, and recommend the administration of RhIG (assuming that the pattern of anti-C plus anti-D was caused by anti-G).
In the second example, in figure 7, anti-D is present in the adsorbed serum and would be identified on testing that serum, so the technologist would not recommend RhIG (though the presence of anti-D opens a whole new discussion about management of the pregnancy, etc).
Gasp! Wheeze! Choke! Sputter!
Seriously, you are still here? Wow, thanks! I apologize for the length of this post, but I felt it was important to be more thorough than most regular blood bank references, because so many people don’t understand this topic.
Here are the take-homes:
- G is an antigen present whenever D and/or C is present
- Anti-G will react against D+ RBCs, C + RBCs, and D+C+ RBCs
- Anti-G is pretty easy to manage in most transfusion patients: Almost all D-negative RBCs will be compatible
- Identifying anti-G is most important for prenatal patients and will make all the difference in getting such a patient the RhIG they need, or saving them from an RhIG dose if not needed
- Teasing out a patient’s antibody specificity is time-consuming, but is valuable for ensuring RhIG is used when indicated, and not unnecessarily
- Adsorptions and double adsorptions, oh my!
I look forward to your further questions and comments on this tricky topic in the comments section below.












Sooooo glad to be retiring in 21 months! As a generalist with blood bank duties, the thought of more is getting old.
Thanks for your dedication to educating those who need to be aware.
You are a treasure.
Sharon, you are too kind! “Treasure?” No, not at all, but thanks for understanding that I am dedicated to education. Please enjoy your 21 months before retirement! As I’ve said in other forums, I hope that now and moving forward (even after retirement) that you will never, ever stop learning!
Thanks Malcolm that was very good explanation. we recently have patient with Anti-G+ Anti-D. In terms of quantification would perform this process every time you received the sample?
Another superb addition to education Joe, although I will never understand why you scientists in the States bother with the elution!!!!!!!!!
I too will be retiring soon (31st October 2016), but I will NEVER stop learning; it is just too much fun.
Just joined this site. Now I see Malcolm Needs name here I know it must be a worthwhile read. Enjoy your retirement, Malcolm.
Malcolm, I’m really interested to hear how a G workup differs in your lab! Fill us in!
Have a great retirement Malcolm. You beat me to it by 11 months, if I make it to September 2017. What will we serologists do in our dotage if we don’t have wonders like anti-G to deal with?
So enriching.With a master in Immunology and looking forawrd to sitting for the ASCP SBB exams,there is so much to learn, new and exam oriented details.
Thanks so much.
Greetings!
Great subject! We once had an AABB Reference proficiency for anti-G. Half the participants called the anti-C which wasn’t supposed to be there and failed and it was ungraded.
I do have a question about how one would follow a prenatal patient with an anti-G, and/or anti-D, and/or anti-C. Normally we do concurrent titers (Past and present samples)using a heterozygous cell of the offending antigen for weakest expression. A change between present and previous samples indicate the HDN is getting worse.
HOWEVER..There appears to be no cells for D and C that don’t also have G. How does one know what they are titering, the D , the C, or the G? Can we just pick two cells, DG (R2r), and CG (r’r), and titer them over the course of the pregnancy. Any increase would be significant regardless of the culprit? What do folks do?
Thanks for the site.. It’s really cool!
Dee, I’m not ignoring your excellent question! I will get to it as soon as I can. Thanks for your patience, and thanks for calling the site “cool!”
I am interested in the answer to this question regarding how to follow a prenatal patient with an anti-G, and/or anti-D, and/or anti-C. Please let me know if this question has already been addressed and where I can find the answer or what suggestions you have. Thank you in advance!
Hi! We are blood bank girls from Madrid La Paz University Hospital. Congratulations for your work!
We have 2 questions regarding Anti-G
22weeks pregnant lady rr, with a D positive fetus.
1- We have adsorbed with C-D+ red celi the serum of the patient and We have anti-C in remaining serum and We elute something that could be Either anti-D or antiD+G
2- We skip the Second elution and We performed the D diferentiation and We adsorbed the patients serum with C+D- red cells. We élute as you explained anti-G +anti-C but in the serum and this is the questions We have an antibidy that aglutinate all the cells D positive and Also the red cell r’r’. This would means that the patient has anti-D +G in the remaining serum or just anti-G that We left? I can assure you that the adsorption was thourouly performed but We do not Kong with this result whether the patient needs anti-D prophylaxis
Thanks a lot!!
In our lab, we end up with an eluate made from adsorbing cells which contains anti-G… lots of my co-workers want to perform titration studies on this eluate, but I am hesitant, as eluates are very variable in strength, depending on how much buffer is used, etc. Any ideas or thoughts?
I’m not sure about performing titers on an eluate, Sara. I personally would be concerned about artificial concentration of the antibody. I’d be happy to hear opposing views, but that would be my concern.
This is my first week working/training in a trauma 1 blood bank. I have two bookmarks on my work computer so far: my e-mail, and BBG. Cool blog, thanks for your effort!
I’m honored, Vince! Good luck!
you are too good….
transfusion medicine is blessed in having a person like you
salute you sir..
Excellent Joe. Your are a very very good teacher. thank you very much.
You are very kind, Dr. Khan. Thank you.
Joe
Dr Joe i must say you are an excellent narrator….excellently clearified all the ambiguities in a quick review..thanks a million.
Thanks so much! I’m very glad it was useful to you.
Joe
Dear Dr. Joe,
That was great, presenting the complex part with such a simplicity.
Kindly throw some light on possibility of having anti-G in RH D-positive female.
Is there any utility of Rh Immunoglobulin if the mother is weak D or D positive?
Thanks, Naveen. My responses to your questions below:
Q1: Almost always, D-positive people are G-positive people, so anti-G should not be present. There are rare examples, though, of people that are D-positive but G-negative, so a person like that could theoretically make anti-G.
Q2: For weak D’s that are at risk for making anti-D with exposure to D, RhIG is recommended. Most recommend genetic (molecular) D typing to know for sure if the mom is at risk. For regular D-positive moms (ones without a D variant), there is no utility.
-Joe
Dear Dr. Joe
Could you explain for us type 1/2/3 weak D’s, what is the difference between them, does they gave positive reaction with AHG phase?
Thank you very much for your interesting presentation.
Ash, I did this on the BBGuy 5 in 10 session yesterday on Facebook Live. Thanks for sending the question!
-Joe
Thank you very much for your time and efforts. Could I say safely that people on regular transfusions with D variants should be genotyped?
That is the current recommendation in the U.S., yes. This issue is really better for the discussion on the Rh genotyping podcast episode.
-Joe
Excellent Joe. Your are a very very good teacher. thank you very much.sir
Thank you, I’ve always had the doubt, my blood type is O, RH-, Anti G, and I asked what it was. and was told just a variation, not to worry.
Absolutely brilliant article. Can you shed light on why the literature (and my very limited personal experience) indicate that anti-G should be suspected when the strength of agglutination is greater with D=C+ reagent cells than with D+C=?
Well, Adam, the deal is simply that anti-G typically gives a lower titer of positivity with D+ cells than real-live anti-D. What I don’t know if ANYONE knows is the “why” behind that. I asked two world-class, brilliant immunohematologists that very question (Sue Johnson and Dr. Connie Westhoff), and they suspected that the reason is that many people with anti-G are actually immunized with C+D- cells rather than the other way around. However, I I’m not sure that anyone knows for sure. I’d summarize the approach this way: If a person appears to have anti-D and anti-C (anti-C+D), and the titer against D+ cells is less than the titer against C+ cells (the opposite of what is usually seen), one should suspect that anti-G is present rather than anti-D. Such a finding is not definitive, but is most often true.
hello sir
i am a blood bank technologist and last week i received a case of Rh negative it was a male 40 years old who received packed red blood cells in 2014 and the patient developed Anti-D and Anti-C my question is: is it possible for this male patient who is A negative to have an Anti-G or its not possible in male cases
Anti-G is totally possible in the case you are describing! Male or female is irrelevant in whether or not a person forms anti-G (aside from the pregnancy issue, of course!). In the setting you describe, if the man received blood from a r’r donor (D-neg, C-pos), he could certainly have formed anti-G. The article above describes how this could happen.
-Joe
Hello,
Here is case that has always been in the back of my mind. I’m just starting to understand Anti-G so bare with me if it sounds like I have no clue what its all about…pregnant female laboring a still born baby at 34 weeks gestation. Early labor but baby was ok until just a couple hours into labor when it started showing distress and a c-sec was done, baby was not alive upon being born. Mother is B positive with a weak D expression and C-. After running panels showing an apparent anti-D we questioned the nurse for more patient info. This was her first pregnancy, no RHIG given ever. Patient had a splenectomy about 10 years before this and was transfused with platelets but not red cells. After more specific phenotyping by a reference lab it was determined she had an Anti-Goa (not sure what the “oa” really is.) Doctors didn’t know if the antibody caused any harm to the baby. Patient returns about 4 years later with premature labor on a baby that was born alive but had a NICU stay due to jaundice. Baby’s red cells tested positive for G and was Rh+. All that being said, should this patient have been given RHIG? What special precautions can be taken if the patient needs blood transfusions in the future? Should any special care be taken for future pregnancies?
Massy, anti-Goa is not the same as anti-G. Goa is an uncommon form of the D antigen that is actually a variant D antigen (what we used to call a “partial D”), so the discussion is very different than that in this particular blog post. The antigen has gone by different names, including “Gonzalez” (that is the Go part), “DIVa,” and what we usually call it now: “Rh30.” It’s interesting to note that the patient you mention showed “weak D expression,” because if she carried that particular antigen, it has been reported to have increased expression.
Anyway, all that to say that the story is more complicated than it seems at first glance. There’s no way for me to tell exactly what happened without more test results from both mom and baby. I can say that it seems unlikely that anti-G was the issue. Thanks for writing and forcing me into the dim recesses of my memory for the Rh30 discussion!
-Joe
Love you lectures! Make it so easy for me to understand! And it is so fun to read – “…running for the ibuprofen…”. Lol, Dr. Chaffin, you are one of the BEST!
Well thanks, Eric! I’m glad you could have a smile while learning something new.
-Joe
Hello there just would like to say I really love you lectures. I am currently an MLT studding for the MT exam. Do you know if there is a Microbiology guy out there
Lorie, thank you for your kind words. For Micro, the best review site I know of is run by my friend Dr. Margie Morgan (http://www.microbeswithmorgan.com). She’s awesome, and is a great microbiology teacher (and THAT comes from a person who can’t stand micro!). I wish you the best.
-Joe
Is it possible for a person to have three seperate antibodies? He or she not only has an anti G but also a seperate anti D and a seperate anti C? Thanks
Possible? Yes. Not common, though.
-Joe
Greatings from Portugal! Great blog, already added to my bookmarks!
Today we got a pregnant woman that on antibody identification panel of 3 and 11 cells appeared Anti-C and Anti-D, and we started to wonder if it was an Anti-G or an Anti-D + Anti-C. Problem is we don’t have test RBCs G+ in our laboratory…
From what I could understand from your post, it’s only possible to find Anti-G if you have test RBCs G+ ; is there any way that we could separate Anti-G with only D+ and C+ test RBCs?
Great explanation – love your style!
Dear Joe: Very interesting. I am in my 68th year of working in a lab and I just learned more from you. When we started we called the GOa a mutation of D+.
I’m honored to have provided something helpful, sir! Thanks for writing.
-Joe
Quote:
“NOTE: OK, to be completely honest, there are a very small number of D+C- people that lack G, as well as a very small number of D-C- people that have G.”
I just had antibody screen on a 10 week pregnant patient return with anti-D and anti-C antibodies. She is D positive. Could this be explained by her being one of these rare D antigen positive, G antigen negative individuals?
Sure, that’s possible, but there are other possibilities. It’s more likely that the person has a D variant (partial D) and has formed anti-D as well as anti-C.
-Joe
Do we have any data about G antigen expression on various phenotype. Which one have highest and which one have lowest.
your articles are truly wonderful. concepts are well explained. thank you very much for all the efforts.
Very interesting reading.
I have a little story/question I hope you can help me with.
When I was pregnant with my third baby the doctors found out I had developed anti-G.
They took blood tests every 2. week to titre it (titer varies from 4 – 8)
It did not increase, and they thought that the baby would not be effected.
They where wrong. My baby got anemic just a few hours after birth, jaundice and signs of infection. We struggled for a year because she didn’t make enough red blood cells.
Why did this happened? Where they not able to titre the anti-G?
Could this have something to say for my daughters future?
Thank you!
Thank you for writing. I’m very sorry to read what your daughter and family went through. Unfortunately, there is just no way I can tell what happened without having much more information about the case. I do not offer consultation for issues like this through my educational web site. I would suggest discussing with local medical personnel. I wish you the best.
-Joe
Thank you so much for your reply. I really appreciate you taking your time.
The medical personnel where I live have little to no knowledge about the Anti-G. So I’m trying to do my own research.
She is nearly two now and she seems fine now with just iron supplements, I’m just so curious 🙂
– C.
Dr. Chaffin, can you please make a video lecture on this?? I love to watch the youtube series on bloodbank and it would be so helpful if there were a video lecture on this topic!
This is so amazing! Would have taken me a while to digest this in text form while this is very simple and easy to understand. Thank you for the time and effort !!!
Thank you Dr chaffin it was great explanation .
Thank you Dr Chaffin. Also a helpful article in far away Poland
Today, I got a pan reactive Ab ID panel of an O neg pregnant woman. Some 4+ reactions of the pan reactive panel suggest the presence of an Anti-D and Anti-C. And there’s this G to consider too; the autoantibodies interfering with the test; the possible other alloantibodies that’s hiding. Crazy blood bank…
I called our supervisor. Prepared cells for allo-adsorptions, endorsed the case to the next shift, and went home. lol.
Thank you for this article, Dr. Chaffin! You don’t have any idea about how you make my life easier. <3
keep safe!
Excellent presentation Joe! A little confusing adsorptionA and elutions explained very well . Very much helpful in solving the complex problems .
Thank you for the kind words!
-Joe
Thank you for your article Dr. Chaffin.
I have a little question.
What is the disadvantage of using parallel adsorption-elution tests (using R2R2 and r’r cells) with respect to the double adsorption method described in the article?.
Thank you
Figure 5 would be “adsorbed eluate”, not “adsorbed serum” since you are using the eluate from the first D+ adsorption with serum/plasma. The discussion is correct, but the picture is misleading. So tricky!
Dear Dr. Chaffin,
Your article (a slightly shorter version than this one from 2016) helped me a lot in identifying my first anti-G antibody in 2014. in a pregnant woman who had anti-G and anti-C.
From then until today, I have proven this antibody in a female blood donor (C+G), one patient (C+G) and two other pregnant women.
One pregnant woman with D+G, and the last case these days of a pregnant woman with anti-G and a newborn with DAT positive without clinical manifestation of HBN.
I think that when identifying anti-G antibodies in pregnant women, it is enough to perform one adsorption and elution technique to confirm the presence of anti-G, and then it is mandatory to perform “D-differentiation” and also anti-C differentiation.
Erythrocytes (R2R2 or r’r) for adsorption should be chosen according to the strength of agglutination in the crossmatching.
Thank you and best regards.
Dear Dr. Chaffin,
Have auto anti D and C (anti-G) antibodies been identified in D+C+ individuals?
Case study:
29 year old female pregnant with 7 child, no prenatal care, all home births. Unknown if or how many miscarriages. She is B Negative never had Rhogam. Positive antibody screen Anti D and Anti C identified (or G) she is negative for C and E.
Titer Anti D 1:512
Titer Anti C 1:32
Thoughts?
Because the D titer is so high and the C is not does this rule out the likelihood of Anti G?
Thank you for this information anti-G. Just yesterday I had a patient I identified with anti-D, anti-C and anti-K. Knowing a little bit about anti-G, I contacted the provider to get additional samples for reference lab testing to check for that since her last RHIG was 10 years ago with her first pregnancy, she is pregnant now and has not yet received RHIG, and she had a transfusion a few years ago, so it kind of doesn’t make sense that she would form anti-D (unless the transfusion was with Rh+ blood). It will be interesting to see the reference lab work up results. Thank you Dr Chaffin for enlightening me to this important distinction and its importance in OB cases.
Hi Mike. Let me know how it comes out. The history you describe certainly makes me wonder about the possibility of anti-G. I’m glad the article was helpful to you!
-Joe