On September 30, 2019, the United States FDA released the long-awaited final guidance regarding bacterial contamination in platelets. Pat Kopko is here to help you understand what to do now!

Dr. Pat Kopko
The Final Guidance is Here!
On 30 Sep 2019, FDA ended the suspense and released their “final guidance” on how U.S. blood collectors and transfusion services should approach detecting (or deactivating) bacteria in platelets stored at room temperature. The guidance looks complicated at first glance, so Dr. Pat Kopko joins me to summarize what we see.
How to Listen to this Episode
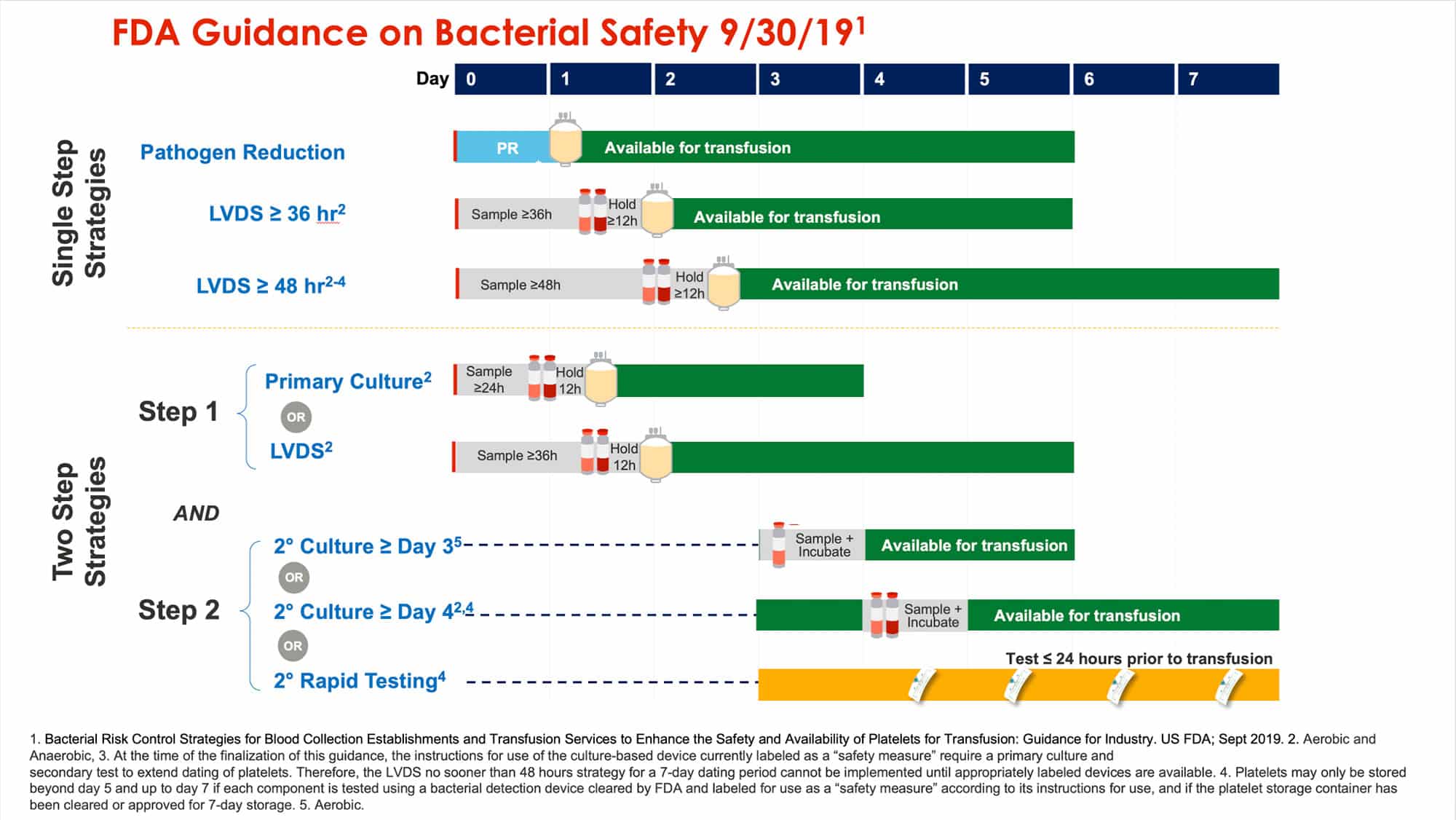
Please use the link below (or click here) to download the guidance, since this works best when you see what Pat and I are describing. You can also refer to the image below, generously provided by Cerus, that summarizes the options graphically. Finally, understand that we ARE NOT giving you advice on which of the options you should choose. We both believe that there is no “one size fits all” strategy, and you will need to evaluate what works best for you and your blood supplier over the next 18 months before the deadline of March 2021.

Dr. Pat Kopko
The Final Guidance is Here!
On 30 Sep 2019, FDA ended the suspense and released their “final guidance” on how U.S. blood collectors and transfusion services should approach detecting (or deactivating) bacteria in platelets stored at room temperature. The guidance looks complicated at first glance, so Dr. Pat Kopko joins me to summarize what we see.
How to Listen to this Episode
Please use the link below (or click here) to download the guidance, since this works best when you see what Pat and I are describing. You can also refer to the image below, generously provided by Cerus, that summarizes the options graphically. Finally, understand that we ARE NOT giving you advice on which of the options you should choose. We both believe that there is no “one size fits all” strategy, and you will need to evaluate what works best for you and your blood supplier over the next 18 months before the deadline of March 2021.
About My Guest:
Dr. Pat Kopko is a graduate of the Loma Linda University School of Medicine in Loma Linda, CA. She did a residency in anatomic and clinical pathology at Loma Linda, followed by a transfusion medicine fellowship at Cedars-Sinai Medical Center in Los Angeles. Dr. Kopko is a Professor of Pathology at the University of California, San Diego, where she serves as Director of Transfusion Medicine and Associate Director of the Immunogenetics and Transplantation Laboratory. Her research interests center around transfusion reactions, particularly Transfusion-Related Acute Lung Injury (TRALI), currently the leading cause of transfusion-related mortality in the United States. Dr. Kopko has published extensively on TRALI and other topics, including platelet refractoriness, transfusion in ABO-incompatible HPC transplantation, and blood transfusion practices.
DISCLAIMER: The opinions expressed on this episode are those of my guest and I alone, and do not reflect those of the organizations with which either of us is affiliated. Neither Dr. Kopko nor I have any relevant financial disclosures.
The image below is generously provided by Cerus (special thanks to Buddy Nguyen).
Further Reading:
- United States Food and Drug Administration, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion.” Released 30 Sep 2019 (accessed 10 Oct 2019)
Thanks to:
- Samantha Chaffin, Design and content consultant
Music Credit
Music for this episode includes “Cuando te invade el temor” and “Reflejo,” both by Mar Virtual via the Free Music Archive. Click the image below for permissions and license details.










Trackbacks/Pingbacks